Forschung
Unsere Mission: Die Identifizierung neuer und effektiver Behandlungsmöglichkeiten für betroffene Kinder durch die Entwicklung innovativer Biotechnologien und die Anwendung neuartiger Forschungsmethoden.
Forschung
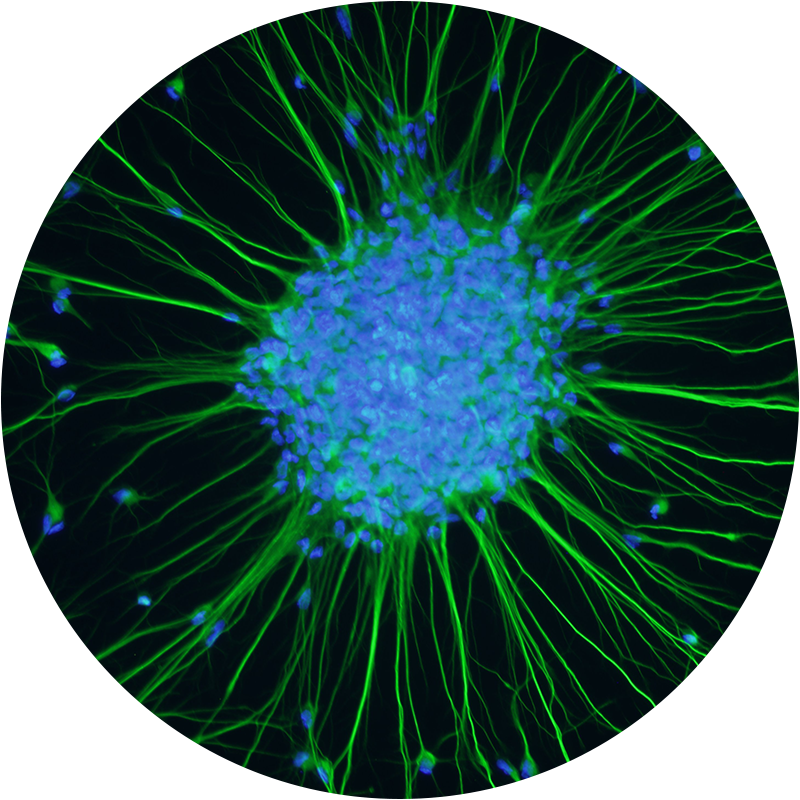
Einführung
Gehirntumore stellen in Deutschland die häufigste Todesursache im Kindesalter dar.
Wirkstoff-Screening
Um den Effekt neuer Wirkstoffe auf DIPG Zellen zu untersuchen, werden in unserem Labor jede Woche Millionen von Tumorzellen mit verschiedenen Substanzen behandelt.
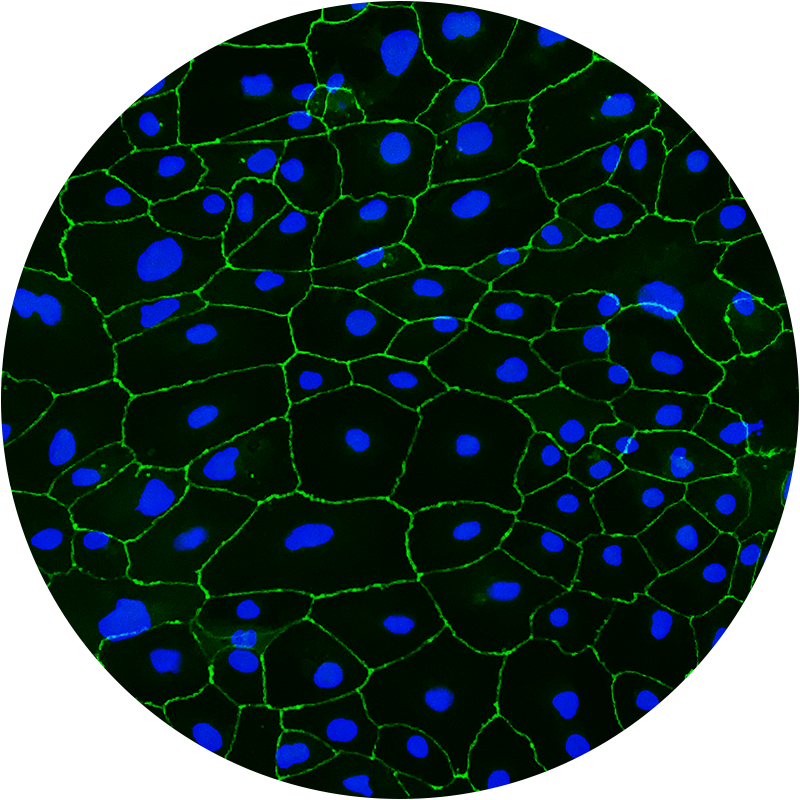
Blut-Hirn-Schranken-Modell
Das Gehirn des Menschen ist durch eine besondere Barriere geschützt: die so genannte Blut-Hirn-Schranke (BHS).
Sie dient als Barriere zwischen dem im Körper zirkulierenden Blut und dem zentralen Nervensystem und reguliert den Stoffaustausch im Gehirn. Diese Schutzfunktion führt jedoch auch dazu, dass viele Medikamente nicht oder nur in geringer Konzentration ins Gehirn gelangen können. Unsere Arbeitsgruppe hat ein neuartiges Labor-Modell der menschlichen Blut-Hirn-Schranke entwickelt, welches mit bisher unerreichter Vorhersagekraft Wirkstoffe in BHS-gängig oder BHS-ungängig einteilen kann. Wirkstoffe, die in unseren Screenings als effektiv gegen DIPG eingestuft wurden, können so auf ihre BHS-Gängigkeit hin untersucht werden. Informationen über die BHS-Permeabilität von Medikamenten sind von unschätzbarem Wert für die Beurteilung eines möglichen klinischen Einsatzes einer Substanz. Selbst wenn Substanzen sehr effektiv gegen DIPG-Zellen wirken, ist den Patienten nicht geholfen, wenn dieser Wirkstoff den Tumor im Gehirn nicht erreichen kann.
Toxizität
Viele Wirkstoffe, die in der Lage sind, Tumorzellen abzutöten, haben auch erheblichen Einfluss auf gesunde Zellen des menschlichen Körpers.
In der Krebstherapie äußert sich dieser Effekt auf nicht maligne Zellen in Form von teils schweren Nebenwirkungen. Wirkstoffe, die nicht gezielt genug auf Tumorzellen wirken, führen in der Klinik häufig zur Dosislimitierung. Das bedeutet, dass Patienten aufgrund von Nebenwirkungen nicht die Dosis erhalten können, die nötig wäre, um den Tumor aktiv zu bekämpfen. Da es sich bei DIPG-Patienten in aller Regel um Kinder handelt, ist es besonders wichtig, dosislimitierende Toxizitäten möglichst früh zu identifizieren. Zu diesem Zweck erstellen wir Zellmodelle der wichtigsten Organsysteme des Menschen. Mit Hilfe von pluripotenten Stammzellen können wir so menschliche Herzmuskelzellen, Nierenzellen, Leberzellen und viele andere Zellarten in großer Anzahl generieren und den Effekt von vielversprechenden DIPG-Wirkstoffen auf diese nicht malignen Zellpopulationen untersuchen. Ein besonderes Augenmerk liegt dabei auf dem Effekt der Wirkstoffe auf Nervenzellen. Da wir Medikamente identifizieren, die in der Lage sind, die Blut-Hirn-Schranke zu überwinden, ist es besonders wichtig, eine Neurotoxizität dieser Wirkstoffe auszuschließen, da diese ungehindert ins zentrale Nervensystem gelangen können. Mit unseren Toxizitäts-Analysen generieren wir wertvolle Informationen für behandelnde Ärzte und identifizieren Therapien mit weniger dosislimitierenden Nebenwirkungen.
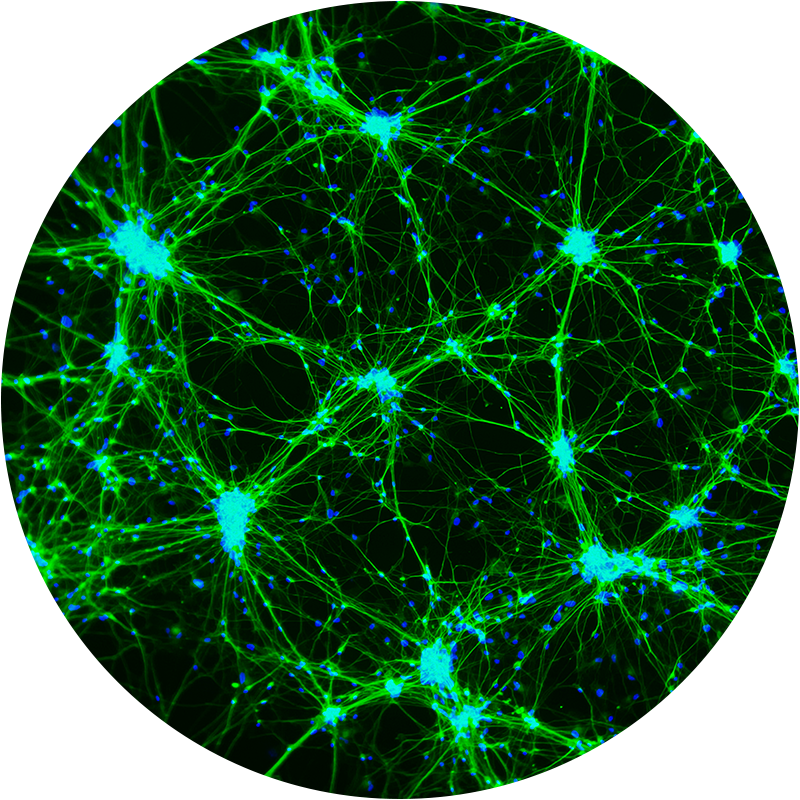

Wirkstoffkombinationen und Resistenzen
Es gibt konkrete Hinweise darauf, warum die aktuell zur Behandlung von DIPG eingesetzten Medikamente keinen entscheidenden Erfolg haben.
Komplexe Tumormodelle
Bei DIPG handelt es sich um einen komplexen Tumor.
Er wächst diffus in das umliegende gesunde Gewebe ein und interagiert mit diesem. Daraus ergeben sich für die einzelnen Tumorzellen unterschiedliche Umgebungsbedingungen. Zudem gibt es innerhalb eines DIPGs verschiedene Tumorzellpopulationen. Um neue Therapieoptionen finden und testen zu können, müssen die Tumorzellen auch im Labor Umgebungsbedingungen ausgesetzt werden, wie sie im menschlichen Gehirn herrschen. Eines unserer Projekte beschäftigt sich daher mit der Integration von DIPG Zellen in „Mini-Gehirne“. Diese Mini-Gehirne sind dreidimensionale Strukturen von ca. 5mm Größe und werden aus menschlichen Stammzellen generiert. Die Mini-Gehirne beinhalten zahlreiche Zellarten des menschlichen Gehirns und erzeugen so optimale und realitätsnahe Wachstumsbedingungen für Gehirntumorzellen. Mit diesem Modell können wir hunderte von Wirkstoffen testen und neben dem Effekt der Therapeutika auf verschiedene Tumorpopulationen auch den Effekt auf die normalen, nicht malignen Gehirnzellen testen.

Publikationen
Für alle, die sich noch intensiver über unsere Forschung informieren möchten, verlinken wir hier die aktuellsten Veröffentlichungen unserer Arbeit:
The landscape of tumor cell states and spatial organization in H3-K27M mutant diffuse midline glioma across age and location.
Nature genetics, 2022 Dec, 54 (12) 1881-1894
BAF Complex Maintains Glioma Stem Cells in Pediatric H3K27M Glioma.
Cancer discovery, 2022 Dec 02, 12 (12) 2880-2905
Preclinical and clinical evaluation of German-sourced ONC201 for the treatment of H3K27M-mutant diffuse intrinsic pontine glioma.
Neuro-oncology advances, 2021, 3 (1) vdab169
Filbin MG, Tirosh I, Hovestadt V, Shaw ML, Escalante LE, Mathewson ND, Neftel C, Frank N, Pelton K, Hebert CM, Haberler C, Yizhak K, Gojo J, Egervari K, Mount C, van Galen P, Bonal DM, Nguyen QD, Beck A, Sinai C, Czech T, Dorfer C, Goumnerova L, Lavarino C, Carcaboso AM, Mora J, Mylvaganam R, Luo CC, Peyrl A, Popović M, Azizi A, Batchelor TT, Frosch MP, Martinez-Lage M, Kieran MW, Bandopadhayay P, Beroukhim R, Fritsch G, Getz G, Rozenblatt-Rosen O, Wucherpfennig KW, Louis DN, Monje M, Slavc I, Ligon KL, Golub TR, Regev A, Bernstein BE & Suvà ML
Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq.
Science (New York, N.Y.), 2018 Apr 20, 360 (6386) 331-335
